Posts
My thoughts and ideas
Welcome to the blog
My thoughts and ideas
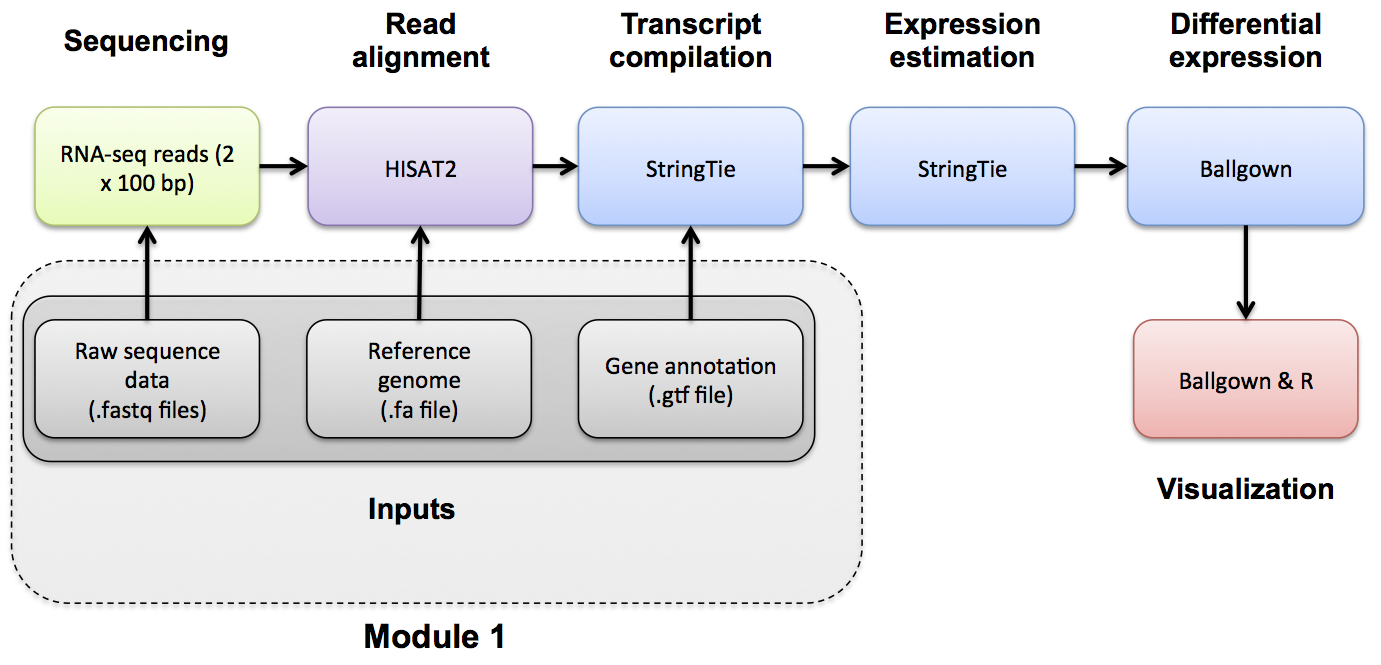
Introduction to bioinformatics for RNA sequence analysis
You can use FastQC to get a sense of your data quality before alignment:
Video Tutorial here:
Try to run FastQC on your fastq files:
cd $RNA_HOME/data
fastqc *.fastq.gz
Then, go to the following url in your browser:
*_fastqc.html files to view the FastQC reportExercise: Investigate the source/explanation for over-represented sequences:
Fastp is a similar alternative tool. QC results for this tool can be produced as follows
cd $RNA_HOME/data
mkdir fastp
cd fastp
mkdir HBR_Rep1 HBR_Rep2 HBR_Rep3 UHR_Rep1 UHR_Rep2 UHR_Rep3
cd $RNA_HOME/data/fastp/HBR_Rep1
fastp -i $RNA_HOME/data/HBR_Rep1_ERCC-Mix2_Build37-ErccTranscripts-chr22.read1.fastq.gz -I $RNA_HOME/data/HBR_Rep1_ERCC-Mix2_Build37-ErccTranscripts-chr22.read2.fastq.gz
cd $RNA_HOME/data/fastp/HBR_Rep2
fastp -i $RNA_HOME/data/HBR_Rep2_ERCC-Mix2_Build37-ErccTranscripts-chr22.read1.fastq.gz -I $RNA_HOME/data/HBR_Rep2_ERCC-Mix2_Build37-ErccTranscripts-chr22.read2.fastq.gz
cd $RNA_HOME/data/fastp/HBR_Rep3
fastp -i $RNA_HOME/data/HBR_Rep3_ERCC-Mix2_Build37-ErccTranscripts-chr22.read1.fastq.gz -I $RNA_HOME/data/HBR_Rep3_ERCC-Mix2_Build37-ErccTranscripts-chr22.read2.fastq.gz
cd $RNA_HOME/data/fastp/UHR_Rep1
fastp -i $RNA_HOME/data/UHR_Rep1_ERCC-Mix1_Build37-ErccTranscripts-chr22.read1.fastq.gz -I $RNA_HOME/data/UHR_Rep1_ERCC-Mix1_Build37-ErccTranscripts-chr22.read2.fastq.gz
cd $RNA_HOME/data/fastp/UHR_Rep2
fastp -i $RNA_HOME/data/UHR_Rep2_ERCC-Mix1_Build37-ErccTranscripts-chr22.read1.fastq.gz -I $RNA_HOME/data/UHR_Rep2_ERCC-Mix1_Build37-ErccTranscripts-chr22.read2.fastq.gz
cd $RNA_HOME/data/fastp/UHR_Rep3
fastp -i $RNA_HOME/data/UHR_Rep3_ERCC-Mix1_Build37-ErccTranscripts-chr22.read1.fastq.gz -I $RNA_HOME/data/UHR_Rep3_ERCC-Mix1_Build37-ErccTranscripts-chr22.read2.fastq.gz
Run MultiQC on your fastqc reports to generate a single summary report across all samples/replicates.
cd $RNA_HOME/data
multiqc ./
Then, go to the following url in your browser:
Move all the FASTQC files into their own directory
cd $RNA_HOME/data
mkdir fastqc
mv *_fastqc* fastqc
Assignment: Run FASTQC on one of the additional fastq files you downloaded in the previous practical exercise.
Run FASTQC on the file ‘hcc1395_normal_1.fastq.gz’ and answer these questions by examining the output.
Questions
Solution: When you are ready you can check your approach against the Solutions.